Utidelone recommended by “2023 CSCO Guidelines for Diagnosis and Treatment of Breast Cancer” as Class I




UTD1 Phase 3 INDs approved by NMPA for NSCLC and BC neoadjuvant; UTD2 Phase 1 INDs approved by FDA and NMPA
Utidelone listed in “China's national reimbursement drug list”

Biostar Pharma, Inc. (US subsidiary) incorporated

Extraordinary general meeting held and share-holding reform completed

Utidelone Injectable NDA approval for the first indication of advanced breast cancer

Pre-IPO financing of 890 million RMB

Utidelone Injectable NDA submission as priority review

Chengdu manufacturing base established for microbial fermentation production and drug product manufacturing

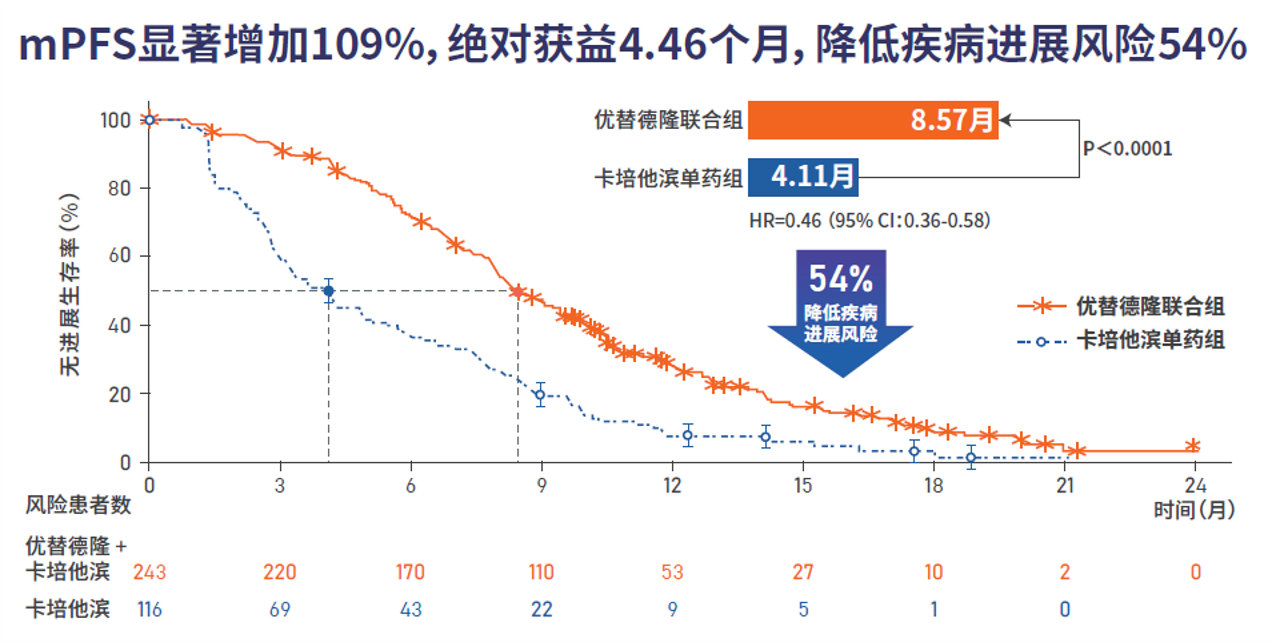
Utidelone Injectable Phase 3 reached the primary endpoint
Wholly-owned subsidiary incorporated in Chengdu

R&D center established covering key biosynthesis platforms

Utidelone Injectable Phase 2/3 IND approved

Utidelone injectable Phase 1 IND approved

Beijing Biostar Pharmaceuticals incorporated in Beijing China


